Many people have questions about how vaccines are developed. This more and more true as emergency vaccines come on the market. Here’s what you need to know about vaccine development.
Vaccine Development
Vaccine development is a closely monitored and rigorous process. All vaccines, even accelerated vaccines, must adhere to all the safety and ethical protocols. Important things to know about vaccine development:
- Vaccines have been studied for more than 100 years.
- Vaccine development is NOT started from scratch – it builds on a strong foundation of what is already known to work and be safe
Scientists understand short and long term side effects because:
- 99.9% of short-term side effects are found within several weeks of vaccination
- Most long-term side effects are found within about 45 days of vaccination
Vaccines are literally the most researched and monitored health interventions in medicine. You can be confident in their effectiveness in preventing dangerous diseases AND their safety.
Vaccine Effectiveness
Vaccines have single handedly saved billions of lives. Need proof? Consider measles, one of the most contagious disease on earth.
- Measles is so contagious that if one person with measles is in a room with 10 unvaccinated people, 9 of them will get sick.
- Before the vaccine, in the US alone, there were between 3 and 4 million cases of measles each year.
- In 2019, there were over 200,000 deaths from measles worldwide in areas with low vaccine rates.
Did you know…
Vaccines prevented at least 10 million deaths between 2010 and 2015 and save about 42,000 lives in the U.S. every year.


The good news?
The measles vaccine works! When children get both doses of the measles vaccine 97% of them will not get the measles.
The bad news?
The U.S. actually eliminated measles in 2000 but because of vaccine hesitancy, cases have been increasing year by year. During the US 2018-19 measles outbreak, there were over 1,200 cases across 31 states. Most cases were in people who were not vaccinated.
Measles is not the only disease that can be brought under control by vaccines. It was a vaccine that finally brought the 2014-15 West African Ebola outbreak under control. For as frightening as Ebola was, just five years later COVID-19 made the world stand still. As of August 2021, 203 million people in the world have gotten COVID-19 – but vaccines are on the scene and as more people get vaccinated, fewer are dying from COVID.
Vaccine Safety
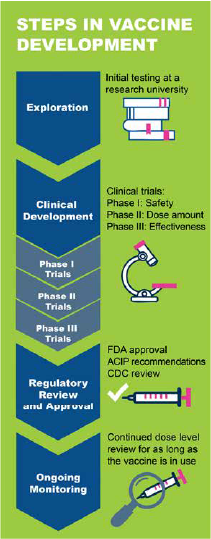
Vaccines are some of the most researched medical interventions on earth. We have been researching vaccines for more than 100 years. And billions of children have been safely protected from serious diseases in that 100 years. By the time a vaccine is approved and is at your doctor or pharmacy it has gone through a rigorous process to make sure it works and is safe. This process can take years and thousands of hours of scientific study. It includes:

Clinical Trial Data: There are usually thousands of people enrolled in clinical trials so there is a LOT of data focused on safety. For instance, Pfizer enrolled 43,661 people in its COVID-19 vaccine clinical trial.

FDA Review and Liscensure / Emergency Use Authorization: If a vaccine is found to be safe and effective during the clinical development phase, it is reviewed by the Food and Drug Administration (FDA) for use in the general public. The FDA will only approve a vaccine if it is safe, effective, and the benefits outweigh any risks. Almost every country in the world has an equivalent to the United State’s FDA, which means vaccine data are reviewed by dozens of regulatory bodies around the world for safety and effectiveness.

ACIP Review and Guidance: Once a vaccine is licensed and approved for use, the Advisory Committee on Immunization Practices (ACIP) reviews all the data and provides guidance on how a vaccine should be used.
ACIP (Advisory Committee on Immunization Practices) is an independent group of vaccine experts from top universities, research institutions, physician academies, and hospitals who evaluate the data to develop the recommendation for who should get the vaccine.
After the ACIP makes its recommendation, the Director of the Centers for Disease Control and Prevention (CDC) reviews the data and ACIP’s recommendation. If the director approves the recommendation, the vaccine is added to the U.S. vaccine schedule.

Ongoing Dose-Level Monitoring: This is the monitoring that takes place after a vaccine is administered and captures any unusual side effects or adverse events. If anything is found, the FDA and CDC evaluate the findings to ensure there isn’t a problem. There are four systems that do this:
- CISA
- VAERS
- Vaccine Data Safety Link
- VSafe (currently only for COVID-19 vaccines)
The truth is that your child’s scrape from a fall on the playground is probably a bigger deal for their immune system than a vaccine. That’s because today’s vaccines use such a small amount of antigen (what helps your body create an immune response). Our 100 years of vaccine research has made us really good at vaccine development. Thirty years ago, vaccines protected against eight diseases and contained more than 3,000 viral and bacterial proteins. Today, vaccines protect against 14 diseases, using only 305 proteins. So for those that are worried that more vaccines means more work on a young immune system, have no fear – we’re using 10% of the proteins we used to give to offer MORE protection.
Win-Win! That scrape from the playground? It introduces FAR more antigens into your child’s body than vaccines. We encounter antigens everywhere – in our food, in the air we breath, and in the surfaces we touch. It’s not a problem because that is what our immune system is designed for. But remember, sometimes germs can take hold in our bodies before our immune systems can work their magic. This is where vaccines come in. So let your child play on the playground. But also get them vaccinated!

Fast facts about emergency vaccine development
One of the biggest concerns we hear when an emergency vaccine is rapidly development is that it happened “too fast”. No so, and here’s why:

Vaccines have been studied and develped for more than 100 years. Scientists do not need to start from scratch when working to quickly develop a vaccine.

In emergencies, the number of staff and amount of resources put to vaccine development is significantly increased. This helps scientists get answers faster.

In emergencies, researchers limit what they study to safety and efficacy (does the vaccine work) in order to get a safe and effective vaccine as quickly as possible. This saves lives. Other things like how the protection works or how long it lasts is learned as the vaccines are rolled out.
Strength in numbers
The COVID-19 Vaccine Phase III clinical trials had tens of thousands of participants enrolled:
- Moderna: 28,000
- Pfizer: 43,000
- J&J (Janssen): 45,000
This is far more participants than regularly developed vaccines have, and means that the safety data is some of the most robust data presented to the FDA for a vaccine.
If you look at the history of vaccines, you know that virtually all long-term adverse effects of a vaccine occur between 15 and 30 days after you get the dose – 45 days at the most.

Dr. Anthony Fauci Director, US National Institute of Allergy and Infectious Diseases

Make sure your staff knows the facts about long term side effects so they can be advocates for facts and science!
© Voices for Vaccines. Excerpts and links may be used by websites and blogs, provided that full and clear credit is given to Voices for Vaccines, with appropriate and specific direction and links to the original content. Parents, providers, advocates, and others may download and duplicate toolkits in print, without alteration, for non-commercial use and with full and proper attribution only.
