I have enrolled in a #COVIDvaccine trial and last week got my first shot.
This wasn’t always my plan, though. I had a surprising amount of hesitancy early on, so I want to share how I worked through it.
As an avid advocate who believes that vaccines save lives, I found myself personally and professionally skeptical about the speed with which Covid vaccines were being developed and politicized. I decided to read about the development of the Covid vaccines and listened to the New England Journal of Medicine’s weekly podcast each and every week. I also found the New York Times’ Covid 19 vaccine tracker helpful.
In addition, in speaking with infectious disease colleagues of mine, I learned that previous vaccine trials for SARS-CoV and MERS-CoV (Covid’s “cousins”) were referenced as a foundation for the science behind many #Covid19 vaccine trials. Here is a nice review of that, by the way.
Following these resources helped me overcome my initial hesitancy that had arisen because of concerns regarding the speed with which these vaccines have been developed. I’ve shared the information I’ve gathered with my patients and my parents using this analogy from a tweet by Dr. Theresa Chapple: “Think if all the women in your family came to clean your house. It would get done efficiently and quickly, but would you look down on the rushed job? It would probably be better than if you did it on your own because you’d get tips from others.”
Think If all the women in your family came to clean your house. It would get done, efficiently, and quickly, but would you look down on the rushed job? It would probably be better than if you did it on your own because you’d get tips from others.
— Dr. Theresa Chapple (@Theresa_Chapple) December 1, 2020
Next was a concern I had about the mRNA vaccine specifically, as it has not yet been rolled out to millions of individuals. Learning more about the history of #mRNA technology and the companies utilizing it has helped ease my concerns. This article in Stat is a particularly good piece. While we still don’t know the possible short/medium/long term side effects of the vaccine–though based on the overall history of vaccine development those effects are likely rare–vaccines have always been about decreasing risk and #COVID19 presents a significant and even deadly risk to many of our communities. Additionally, most proven vaccine-related long-term side effects have shown up within months, not years. If they exist, they will be caught quickly. So I am reassured that we haven’t yet seen any significant number of side effects in thousands of participants who received their vaccine four or more months ago.
When my family, friends, and patients ask me about balancing the risk of getting #COVID19 with what we don’t yet know about its long-term effects my response is to say that the unknown short/long term risk of a mRNA vaccine is still much more acceptable to me than the risk of getting Covid.
Once I felt ready to get the vaccine, I had a decision to make. Should I wait for an early approved vaccine as an approved healthcare worker who was likely to get early priority? Or should I participate in Covid vaccine trial? In the Twin Cities, my employer was the first to begin enrolling participants in a Covid vaccine trial. You may have heard of the AstraZeneca/Oxford trial that had to be paused–that was the one. The other option available to me in the Twin Cities was with Janssen Pharmaceuticals. Both use a more “traditional” approach of a genetically modified virus to teach the immune system how to make a protective response against SARS-CoV-2.
The researchers running the AstraZeneca/Oxford trial realized there had been a mistake in some of the initial doses, with some participants only getting half a dose. Thing is–those participants actually had a better response. Yet it remains that a miscalculation was made. In determining which trial I should enroll in, some might read this and think the answer is obvious: go with the trial that didn’t have to be halted, the one that hadn’t made a mistake, right? Well, to me, those steps–the halting of the trial, the concern for accuracy and safety–told me that those running the trial were not cutting corners and were willing to admit mistakes.
Appropriate transparency is paramount. You know why.
In addition, the AstraZeneca/Oxford trial as one thing in Minnesota that the Janssen trial doesn’t: Dr. Zeke McKinney. Zeke and I go back to medical school where we worked together at the Student National Medical Association, @SNMA, helping fill our fellow students’ gaps in education around #SDOH before it became a more regular part of the curriculum. Dr. McKinney continues that education today as chief medical editor of Minnesota Medical Association’s MN Medicine, a member of @MNAADocs, and a lead investigator of the Minnesota AstraZeneca/Oxford trial. In an article in the Minnesota Spokesman, he spoke about how the Covid trials help protect human rights, and this resonated with me because as someone with family around the world, particularly Cameroon, I also wanted to support a vaccine that could truly be global. Ultra-low freezers and cold chains aren’t going to work in much of Africa.
So knowing the Oxford scientists used their leverage to get AstraZeneca on board with making their vaccine on a not-for-profit basis worldwide, for the duration of the pandemic, and always at cost to low- and middle-income countries, was also a critical factor for me.
Finally, before any first step could be taken I wanted to get my wife on board with this. Given the rising tide of Covid cases throughout November and the fact she is a public health-trained RN this wasn’t too hard, but, still, a requirement. So now I was reassured about the speed of the vaccine development, comfortable with the risk/benefit of mRNA vaccines, if offered, and considering two trials. I decided on the AstraZeneca/Oxford trial because I trusted their scientific process and because Dr. Zeke McKinney wanted to contribute both locally and globally in addition to making a safe and effective vaccine. Along the way I was inspired by several doctors who shared their experiences enrolling in a Covid trial, including one of my mentors
I was also inspired by several docs who shared their experiences enrolling in a #COVID19 trial, including one of my mentors, Dr Dipesh Navsaria, as well as a number of Black women doctors, including Dr. Chris Pernell:
Explaining safety data in plain language is important to building trust around the coronavirus vaccine, @DrChrisMD says.
People who get vaccinated are exposed to the genetic code of the virus that instructs the body to build an immune response, she says. https://t.co/MoE0JhWmcn
— Here & Now (@hereandnow) December 14, 2020
So, on to my Phase 3 clinical trial experience thus far. I submitted a prescreen questionnaire that asked about my baseline health, occupation/exposure risk, age, gender, and race/ethnicity. Based on those answers I was deemed to qualify for participation. I then scheduled an appointment and was sent a thorough consent packet via email. There I learned that I had a two in three chance of getting the actual vaccine versus a placebo, as well as the frequency with which I’d have to check in-person versus virtually and the planned length of the trial, which was two years. I also learned participants get $100 for every in-person visit. Of note, this was not advertised at all.
Because of a change in my schedule I had to delay my appointment. During that time, more info came out about Pfizer/Moderna. I could now wait and likely get a vaccine for sure in months, maybe weeks. However my mind was set. The day came. I showed up to my appointment and answered some of the same questions from the screening. I also asked several of my own: were they doing a half-dose first shot as part of their protocol given the early results? No, I was told. This was not yet planned in the U.S.. Could I get results of my SARS-CoV-2 antibody tests (part of the blood draw they did)? No, they go to a third-party vendor. Did I really need another nasal swab PCR (joking, but that was now my fifth one since #COVID19 arrived. I’m not a fan). Had there been any discussions about ending the trial early if efficacy and safety are significant given the ongoing surge to allow those who received placebo to get an actual vaccine? Nothing concrete, they said. They are monitoring closely and participants can leave the trial when they choose.
So after getting clearance to have my pic taken by one of the study coordinators, my arm was prepped and in went the needle! It 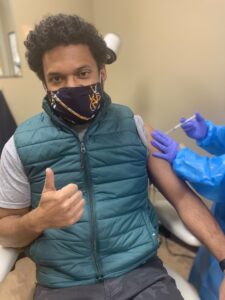 was as routine as my annual flu shot (which I got back in September). Since then I’ve felt some notable fatigue, but otherwise my arm wasn’t even sore. I now answer a questionnaire on an app set up to monitor symptoms and call in any to the clinic. I will then go back in about thirty days for shot #2.
was as routine as my annual flu shot (which I got back in September). Since then I’ve felt some notable fatigue, but otherwise my arm wasn’t even sore. I now answer a questionnaire on an app set up to monitor symptoms and call in any to the clinic. I will then go back in about thirty days for shot #2.
Dr. Nate Chomilo is a general pediatrician and a hospital internist. He is the co-founder of Minnesota Doctors for Health Equity, the medical director for Minnesota’s Medicaid program, an adjunct faculty member of the University of Minnesota Medical School, and a member of the Voices for Vaccines Scientific Advisory Board.